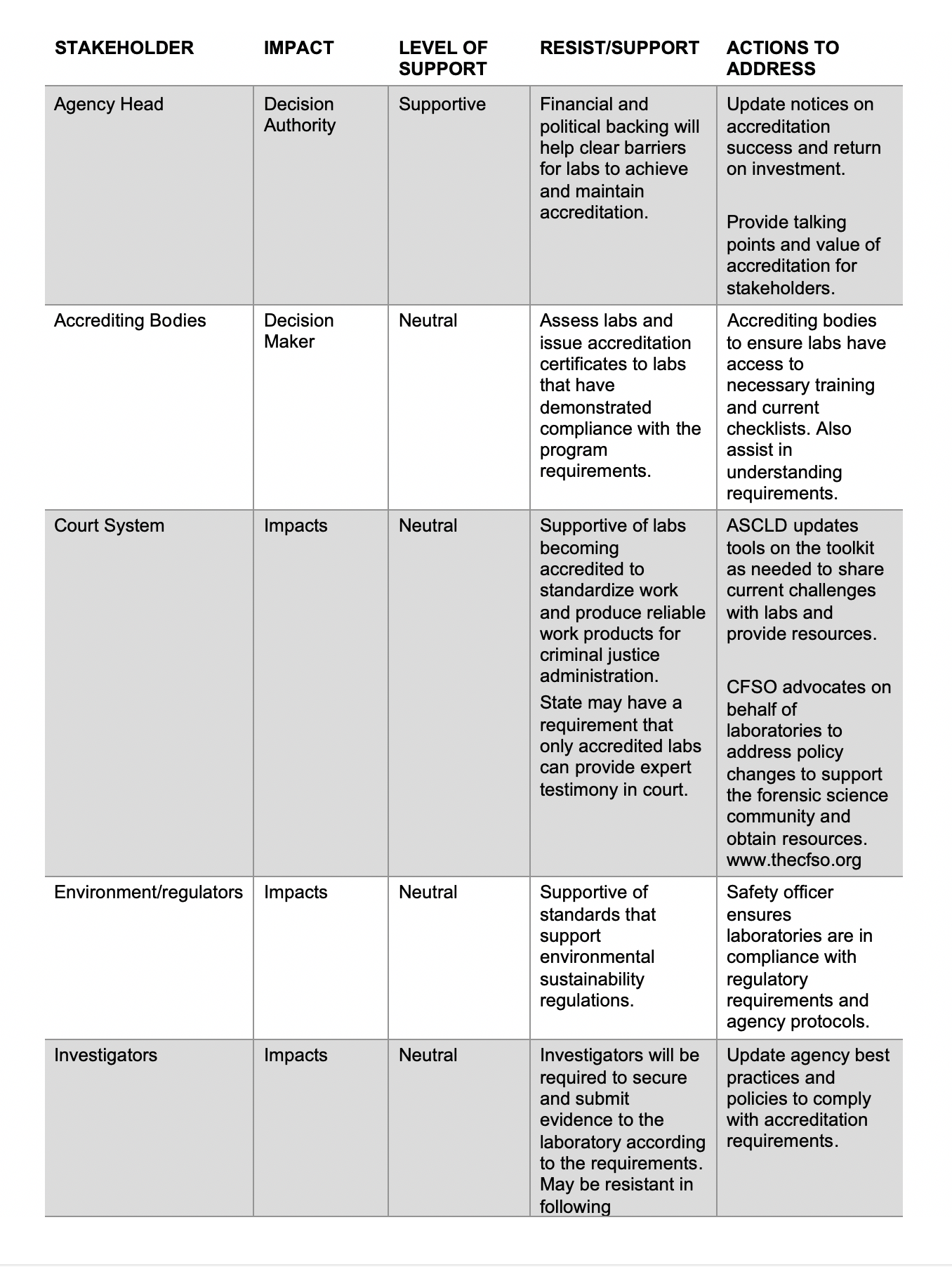
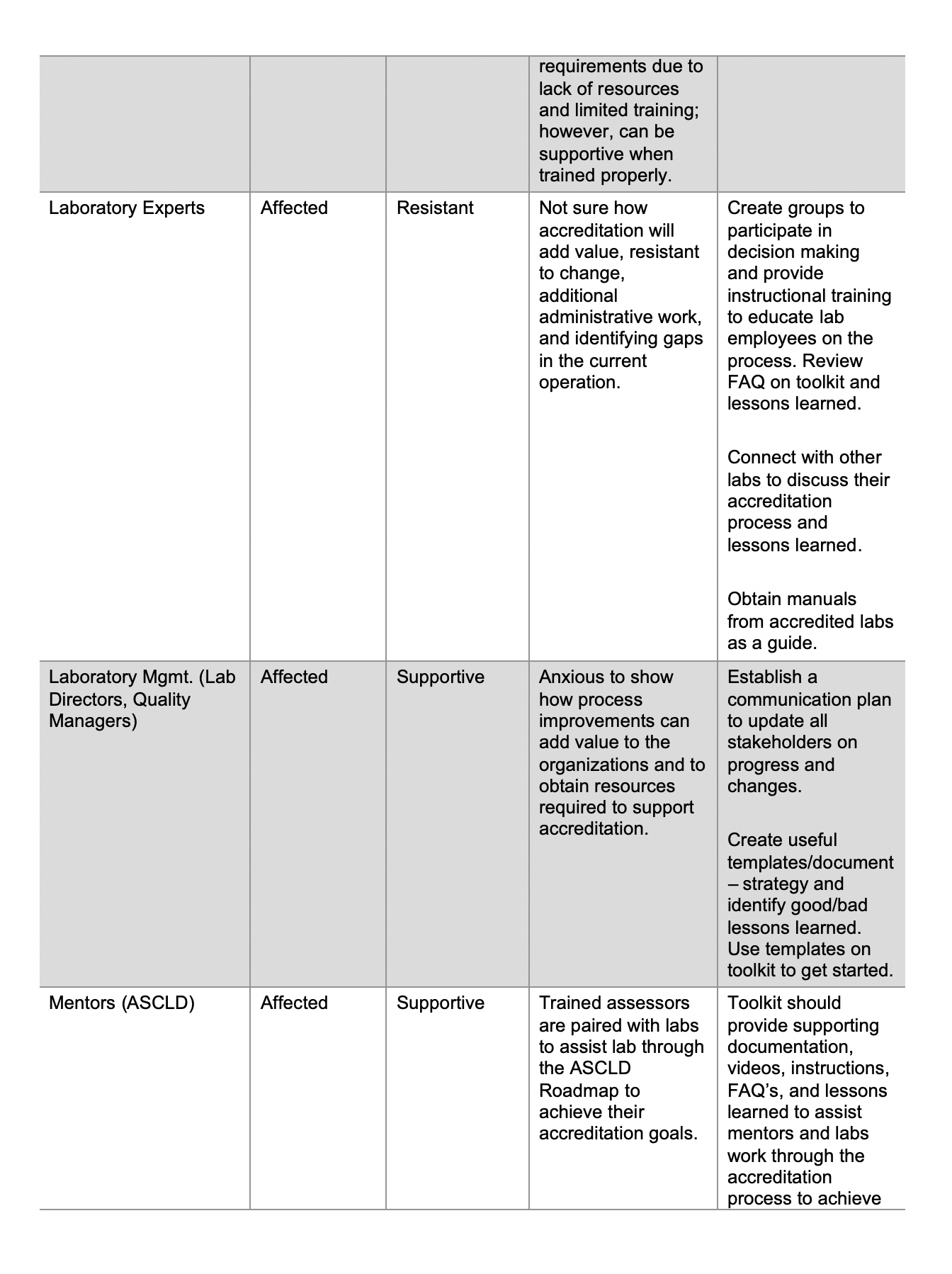
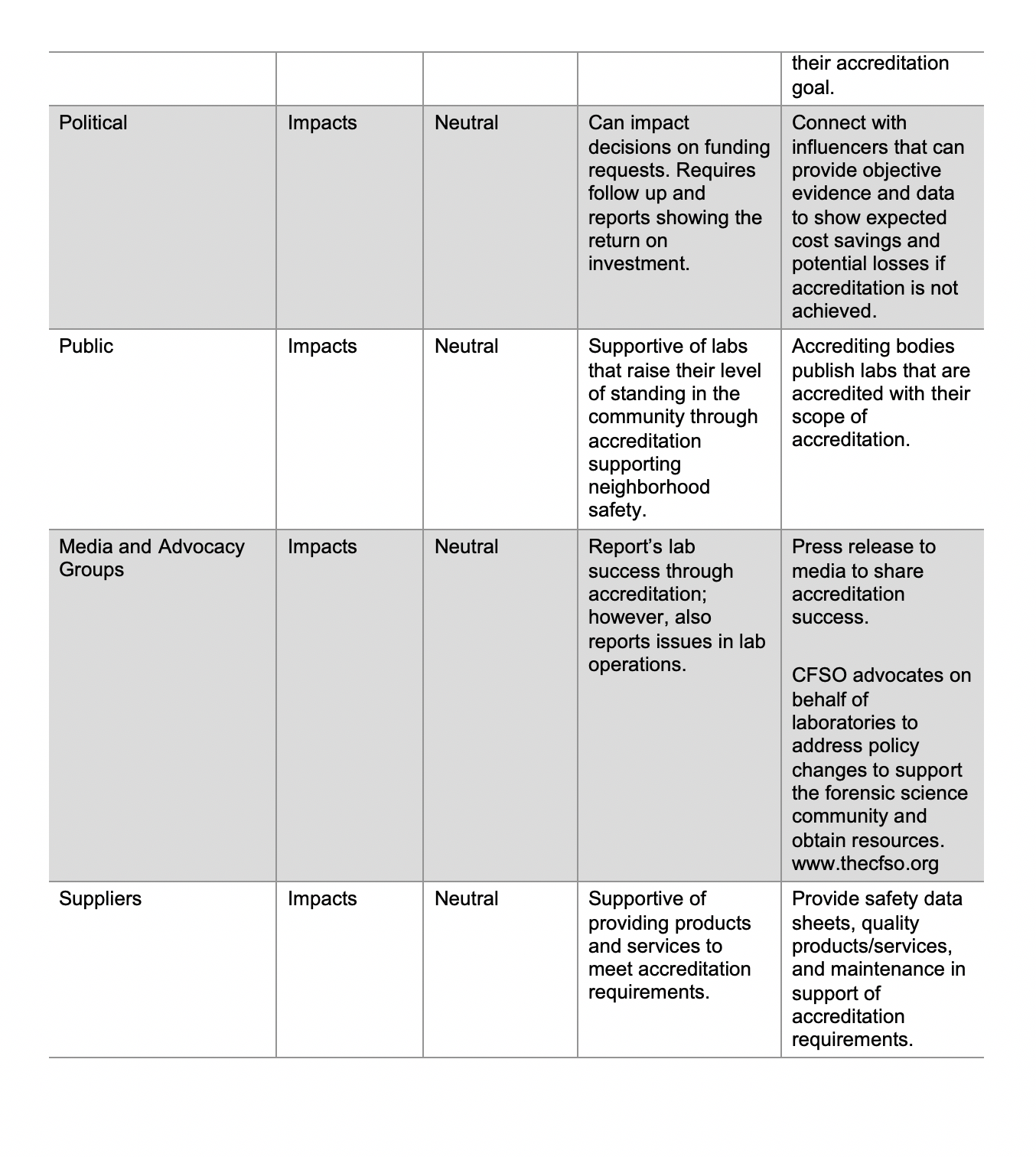
Document control refers to a set of procedures intended to oversee the creation, review, approval, release, revisions, distribution, access, storage, security, and disposal of documents. It is the basis upon which compliance, accuracy, integrity, efficiency, and accountability are built. Remember – when you create a document you need to make sure the correct version is available to all that need access to the document.
Those that perform a quality management system audits, gap assessments and internal audits, a common question that people ask is: “Do I need to control this document?” Here are some considerations to be aware of shown below:
Documents can be in any media:
“Any media” means that documents can be written in paper, electronic, video, audio or similar formats. The documents can be written, pictorials, flow charts, or a combination of these formats.
Documents Need to be Identified:
A simple identifier is the title of the document appearing on the footer or header of each page.
Documents Need to be Approved:
Designate a person or group of people with the authority to determine whether documents need to be controlled and approved. Ideally, the person, such as a document control officer, should be someone that is aligned with the strategic direction of the laboratory and understands the implications and impact of the document.
Documents Need to be Controlled Version Control: Documents must have an identifiable version number visible throughout the document to determine if the correct version is being used. The version can be alphanumeric or by date.
Distribution Control: Documents must be made available to individuals that have a need to know and must be easily accessible.
Keeping Document Control Effective
Here are some examples of keeping document control simple:
Document Stamps: Stamps showing the document status such as: “Reference Only,” “Uncontrolled,” “Not a Controlled Document,” “Master Copy,” etc.
Footer Controls: “Not valid if printed,” “Check system for latest version,” “Not valid after 24 Hours,” etc.
Watermark Controls: Using watermark to notate “Draft”, “Controlled”, “Uncontrolled”, etc.
All these are methods of control but can be misunderstood by those using them. Remember, these are controls; however, assessors will always check for effectiveness. Assessors will consider whether or not the laboratory knows where to find the current version of the document.
What is the Most Common Document Control Issue When Employees are Asked: “What Document do you use?”
Employees may point to a document on their laptop or desktop, or pull the document out from their toolbox or desk drawer, and in most cases, these copies are out of date.
Summary of Document Control
Document Control is used to help the laboratory document processes and procedures that are critical to operate. These documents should align with the strategy and help the laboratory meet requirements in a consistent manner.
Organizing documents on your server? View this Document Tree.